i3- lewis structure|Lewis Structure of I3 : Baguio Nob 25, 2018 — Learn how to draw the Lewis structure of I3- or triiodide ion, a polyatomic ion with 22 valence electrons and sp3d hybridization. Find out its shape, polarity, and how it is soluble in water. The official STL result today, August 1, 2024 (Thursday) Visayas, Mindanao is available here at 10:30AM, 3PM, 5PM, 7PM, 8PM and 9PM. . 2023 following the directive on the use of national games draw results for STL. The use of national draw results was based on PCSO Board Resolution No. 0100 series of 2023 and a .The best sports books have a higher customer rating and many positive reviews. A good sports gambling platform has the following: Promotions and Bonuses. Many players like promos since they allow you to boost your bankroll. Top sportsbooks provide deals such as free bets, welcome offers, reload bonuses, referral bonuses, and VIP perks.
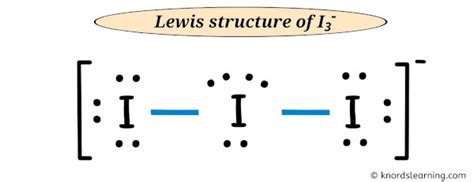
i3- lewis structure,May 25, 2013 — Learn how to draw the Lewis structure for I3- (triiodide ion) with a step-by-step explanation and examples. Find the number of valence electrons, distribute them around the central atom, and .Learn how to draw the Lewis structure of I3- ion, a polyatomic molecule with a negative charge, and understand its bonding nature. Find out its molecular geo.Hul 12, 2020 — This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges .Nob 25, 2018 — Learn how to draw the Lewis structure of I3- or triiodide ion, a polyatomic ion with 22 valence electrons and sp3d hybridization. Find out its shape, polarity, and how it is soluble in water.Learn how to draw the Lewis structure of I3-, the triiodide ion, with 22 valence electrons and a linear shape. Find out the role of lone pairs, the polarity, and the reactivity of I3- in chemistry.
Learn how to draw the Lewis structure for I3- with formal charges and brackets. Watch a video and follow a step-by-step tutorial with examples and tips.Learn how to draw the lewis structure of I3- ion step by step with examples and explanations. Find out the number of electrons, pairs, center atom, lone pairs and charges of I3- ion.i3- lewis structureHun 21, 2023 — Learn how to draw the lewis structure of I3- ion with 22 valence electrons, 3 lone pairs and a -1 formal charge on the central iodine atom. Follow the 5 steps with examples and images to understand the .May 22, 2023 — Learn how to draw the lewis dot structure of I3- ion (triiodide) with 5 simple steps and images. Find out the valence electrons, formal charge, and bond types of I3- ion.
Hun 17, 2024 — Learn how to draw the most stable Lewis structure of I3–, a negative ion with three iodine atoms. Follow the steps to mark lone pairs, formal charges, and brackets on .
Hun 7, 2022 — I3 – structure from wikipedia. How to draw a I 3 – Lewis structure ?. Iodine belongs to 17 th group and 5 th period. It has 7 valence electrons, with expanded shells to occupy any extra electrons apart from the 8 electrons.
Okt 2, 2011 — I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.
The triiodide ion (I3-) is an anion that is formed by combining three iodine atoms. It is a common reagent in chemistry, often used in redox reactions and as an indicator for starch in iodometry. The ion has a linear shape, .2 days ago — The Triiodide ion or I 3-is designated as the Triiodide in Chemistry. It is composed of 3 iodine atoms and is formed by combining the aqueous solution of iodine and iodide salts. There are a few isolated salts of the anion that include ammonium Triiodide ([NH 4] + [I 3] − and thallium(I) Triiodide (Tl + [I 3] −)).It can be well studied and .

Lewis structure of I 3-is given above and you can see how atoms are joint with each other. One iodine atom has located as the center and other two iodine atoms are located around it. . After deciding the center atom and skeletal of skeletal of I3- ion ion, we should mark lone pairs on atoms. Remember that, there are total of .That's the Lewis structure for I3-. This is Dr. B, and thanks for watching. Search our 100 + Lewis Structures (Opens New Window) See the Big List of Lewis Structures : Frequently Tested Lewis Structures Basic CH 4, NH 3, C 2 H 4, O 2, N 2 .Okt 29, 2021 — For the arrangement HCN, the Lewis structure: H–C\(\equiv\)N: The formal charges work out as follows: For the arrangement HNC, the Lewis structure: H–N\(\equiv\)C: The formal charges work out as follows: Both Lewis structures have a net formal charge of zero, but note that the formal charges on the first structure are all zero! .Okt 11, 2023 — The Lewis structure of triiodide [I 3] – consists of three identical iodine (I) atoms. One I atom acts as the central atom while the other two iodine atoms act as outer atoms. There are a total of 5 electron density regions around the central I atom in the I 3 – Lewis structure. Out of the 5 electron density regions, there are 2 bond pairs and 3 .n this lesson, we follow five systematic steps to draw the correct Lewis structure of I3-. Screen capture by Screencast-O-Matic (http://www.screencast-o-mati.
Nob 26, 2020 — An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii.

Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.Set 30, 2022 — BI3 is a chemical formula for boron triiodide. And to help you understand the Lewis Structure of this molecule, we are going to share our step-by-step metho.i3- lewis structure Lewis Structure of I3Abr 17, 2023 — That's three iodine atoms in a row, as an ion. The bonds between the iodines are covalent bonds though.Each iodine brings 7 electrons to the party, and there.Peb 9, 2024 — Steps. By using the following steps, you can easily draw the Lewis structure of I 3 –: #1 Draw skeleton #2 Show chemical bond #3 Mark lone pairs #4 Calculate formal charge and check stability (if octet is already completed on central atom). Let’s one by one discuss each step in detail.Ago 15, 2020 — AX2E3: I3− ; Six Electron Groups . According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair. Because electrons repel each other .Dis 29, 2020 — Here are the steps to draw a Lewis structure. The example is for the nitrate ion. A Lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.The diagram is also called a Lewis dot diagram, Lewis dot formula, or electron dot diagram.
70 More Lewis Dot Structures. I does not follow the octet rule. It will hold more than 8 electrons. Iodine having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons. I 3-is dsp 3 hybridized and contains 3 lone pairs and 2 bonding pairs of valence electrons around the .Ene 30, 2023 — Why are there different ways for the "same" Lewis structure? It depends what you want to show. While the most complete structure is more useful for the novice chemist, the simplest is quicker to draw and still conveys the same information for the experienced chemist. You should learn to recognize any of the possible Lewis structures.
i3- lewis structure|Lewis Structure of I3
PH0 · Triiodide ion (I3
PH1 · Lewis Structure of I3
PH2 · Lewis Structure for I3
PH3 · I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity,
PH4 · I3